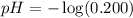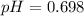Chemistry, 12.11.2019 00:31, Homepage10
For all of the following questions 20.00 ml of 0.200 m hbr is titrated with 0.200 m koh.
region 1: initial ph: before any titrant is added to our starting material
what is the concentration of h+ at this point in the titration?
m
what is the ph based on this h+ ion concentration?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:20, TamB01
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2


Chemistry, 22.06.2019 21:00, lucyamine0
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 22.06.2019 23:30, johnnysteeler9934
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
Do you know the correct answer?
For all of the following questions 20.00 ml of 0.200 m hbr is titrated with 0.200 m koh.
regio...
regio...
Questions in other subjects:


Mathematics, 03.04.2020 04:02

Mathematics, 03.04.2020 04:02


Mathematics, 03.04.2020 04:02



English, 03.04.2020 04:02



 before any titrant added to our starting material is 0.200 M.
before any titrant added to our starting material is 0.200 M. .
.![pH=-\log [H^+]](/tpl/images/0369/7231/37e81.png)