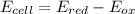Chemistry, 11.11.2019 23:31, mariamakonteh31
In the activity, click on the e∘cell and keq quantities to observe how they are related. use this relation to calculate keq for the following redox reaction that occurs in an electrochemical cell having two electrodes: a cathode and an anode. the two half-reactions that occur in the cell are
cu2+(aq)+2e−→cu(s) and co(s)→co2+(aq)+2e−
the net reaction is
cu2+(aq)+co(s)→cu(s)+co2+(aq)
use the given standard reduction potentials in your calculation as appropriate. ( keq=5.88*10^20)
in the activity, click on the keq and δg∘ quantities to observe how they are related.
calculate δg∘ using this relationship and the equilibrium constant (keq) obtained in part a at t=298k: keq=5.88*10^20
express the gibbs free energy (δg∘) in joules to three significant figures.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:30, janayflowers042
Order the following from smallest to largest atom, electron, quark, proton, neutron, molecule, nucleus
Answers: 1


Chemistry, 22.06.2019 18:00, meowmeowcow
Find the mass, in grams, of 5.00*10^23 molecules of f2
Answers: 3
Do you know the correct answer?
In the activity, click on the e∘cell and keq quantities to observe how they are related. use this re...
Questions in other subjects:

History, 21.02.2021 08:10


English, 21.02.2021 08:10


Mathematics, 21.02.2021 08:10

Arts, 21.02.2021 08:10


History, 21.02.2021 08:10


Mathematics, 21.02.2021 08:10