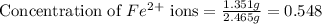Chemistry, 11.11.2019 20:31, ashleyremon
The fe2 (55.845 g/mol) content of a 2.465 g steel sample dissolved in 50.00 ml was determined by tiration with a standardized 0.140 m potassium permanganate (kmno4, 158.034 g/mol) solution. the titration required 34.59 ml to reach the end point. what is the concentration of iron in the steel sample? express your answer as grams of fe per grams of steel (g fe2 / g steel).

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 12:30, MrSavannahCat
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 13:30, kassandrarosario1115
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1
Do you know the correct answer?
The fe2 (55.845 g/mol) content of a 2.465 g steel sample dissolved in 50.00 ml was determined by tir...
Questions in other subjects:

Physics, 27.08.2019 15:30

English, 27.08.2019 15:30



Social Studies, 27.08.2019 15:30

History, 27.08.2019 15:30

Mathematics, 27.08.2019 15:30




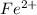 ions is
ions is 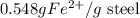
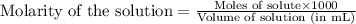
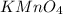 = 0.140 M
= 0.140 M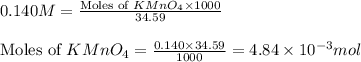
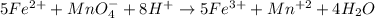
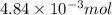 moles of permanganate ions will react with =
moles of permanganate ions will react with = 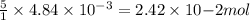 of iron (II) ions.
of iron (II) ions.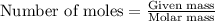
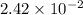 moles
moles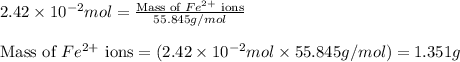
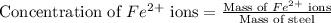
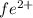 ions = 1.351 g
ions = 1.351 g