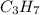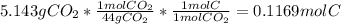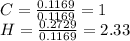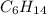A1.678 g sample of a component of the light petroleum distillate called naphtha is found to yield 5.143 g co2 (g) and 2.456 g h2o (l) on complete combustion. this particular compound is also found to be an alkane with one methyl group attached to a longer carbon chain and to have a molecular formula twice its empirical formula. the compound also has the following properties: melting point of -154 c , boiling point of 60.3 c , density of 0.6532 g/ml at 20 c , specific heat of 2.25 j/(g*c), and -204.6 kj/mol use the masses of carbon dioxide, co2, and water, h2o , to determine the empirical formula of the alkane component. express your answer as a chemical formula?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, lydiadmanautou04
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 08:30, myamiller558
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1


Chemistry, 22.06.2019 18:30, bibiansolis
The table lists the lattice energies of some compounds. compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf. the lattice energy increases as the cations get larger, as shown by lif and licl. the lattice energy decreases as cations get smaller, as shown by nacl and naf. the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3
Do you know the correct answer?
A1.678 g sample of a component of the light petroleum distillate called naphtha is found to yield 5....
Questions in other subjects:


Chemistry, 24.05.2020 06:59



Mathematics, 24.05.2020 06:59

Mathematics, 24.05.2020 06:59

Biology, 24.05.2020 06:59

Mathematics, 24.05.2020 06:59

Mathematics, 24.05.2020 06:59