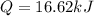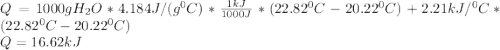Chemistry, 10.11.2019 06:31, angelica9613
A0.3423 g sample of pentane, c5h12, was burned in a bomb calorimeter. the temperature of the calorimeter and the 1.000kg of water contained therein rose from 20.22 degrees celcius to 22.82 degrees celcius. the heat capacity of the calorimeter is 2.21 kj/c. the heat capacity of water = 4.184 j/g c. a. how much heat was given off during combustion fo the sample of pentane. answer = 16.6 kjb. what is the heat of combustion, in kilojoules, per gram of pentaneanswer = 48.6 kj/g

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, giiffnlojd
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 04:00, kichensides
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 09:10, GreatBaconGamer
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 23.06.2019 03:00, KindaSmartPersonn
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
Do you know the correct answer?
A0.3423 g sample of pentane, c5h12, was burned in a bomb calorimeter. the temperature of the calorim...
Questions in other subjects:

Mathematics, 04.11.2021 22:50

Computers and Technology, 04.11.2021 22:50


Mathematics, 04.11.2021 22:50


Engineering, 04.11.2021 22:50

Biology, 04.11.2021 22:50

Mathematics, 04.11.2021 22:50

Geography, 04.11.2021 22:50