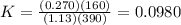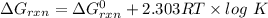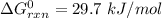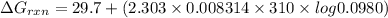Chemistry, 10.11.2019 05:31, elijahjwhite15
Consider the malate dehydrogenase reaction from the citric acid cycle. given the following concentrations, calculate the free energy change for this reaction at 37.0 °c (310 k). δg°\' for the reaction is 29.7 kj/mol. assume that the reaction occurs at ph 7. [malate] = 1.13 mm [oxaloacetate] = 0.270 mm [nad ] = 390 mm [nadh] = 160 mm

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:20, kevinhernandez582
What will most likely happen when two bromine atoms bond together?
Answers: 3


Chemistry, 23.06.2019 06:30, madelineb6243
Which of these describes how heat is transferred by convection* a. sunlight travels through space without the aid of fluids or solids. b. warm air rises and takes the heat with it, eventually, it cools and sinks c. air at the equator rises and sinks at the poles. d. air molecules touch the warm ground, heating them up *not conduction
Answers: 3

Chemistry, 23.06.2019 09:20, weridness80
Which of the following occurs along coasts during the day?
Answers: 3
Do you know the correct answer?
Consider the malate dehydrogenase reaction from the citric acid cycle. given the following concentra...
Questions in other subjects:





Mathematics, 11.03.2020 02:37






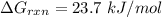
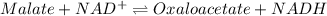
![K =\frac {[oxaloacetate][NADH]}{[malate][NAD^+]}](/tpl/images/0367/7081/e135a.png)
![[NAD^+]](/tpl/images/0367/7081/f5e9f.png) = 390 mM
= 390 mM