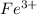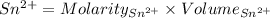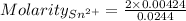Chemistry, 10.11.2019 04:31, xxtonixwilsonxx
Awire weighing 0.250 g and containing 94.75% fe is dissolved in hcl. the iron is completely oxidized to fe3+ by bromine water. the solution is then treated with tin(ii) chloride to bring about the reaction sn2+(aq) + 2fe3+(aq) → 2fe2+(aq) + sn4+(aq) + h2o(l) if 24.4 ml of tin(ii) chloride solution is required for complete reaction, what is the molarity of the tin(ii) chloride solution

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, kkelley9223
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 14:40, elawnnalewis4855
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 22:30, xlebrny7831
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
Do you know the correct answer?
Awire weighing 0.250 g and containing 94.75% fe is dissolved in hcl. the iron is completely oxidized...
Questions in other subjects:







History, 12.04.2021 01:00




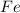 is 94.75 % by mass.
is 94.75 % by mass.
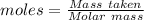
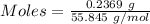
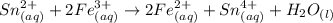
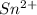 = 2*Moles of
= 2*Moles of