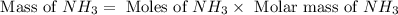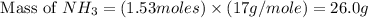Chemistry, 10.11.2019 01:31, maddiehope6140
Consider the following reaction: 2 no(g) + 5 h2(g) → 2 nh3(g) + 2 h2o(g) which set of solution maps would be needed to calculate the maximum amount of ammonia (nh3), in grams, that can be synthesized from 45.8 g of nitrogen monoxide (no) and 12.4 g of hydrogen (h2)? i. g no → mol no → mol nh3 → g nh3 ii. g h2 → mol h2 → mol nh3 → g nh3 iii. g no → mol no → mol h2o → g h2o iv. g h2 → mol h2 → mol h2o → g h2o

Answers: 3
Similar questions

Chemistry, 05.07.2019 23:10, arrow87654
Answers: 2

Chemistry, 25.07.2019 04:00, peno211
Answers: 1

Mathematics, 29.07.2019 19:30, dvnive
Answers: 1

Chemistry, 31.08.2019 07:50, crawford184232323234
Answers: 1
Do you know the correct answer?
Consider the following reaction: 2 no(g) + 5 h2(g) → 2 nh3(g) + 2 h2o(g) which set of solution maps...
Questions in other subjects:

History, 12.09.2019 22:20



Biology, 12.09.2019 22:20

Chemistry, 12.09.2019 22:20

Business, 12.09.2019 22:20


English, 12.09.2019 22:20

History, 12.09.2019 22:20

Biology, 12.09.2019 22:20


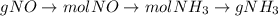
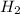 = 12.4 g
= 12.4 g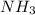 = 17 g/mole
= 17 g/mole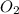 .
.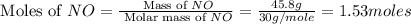
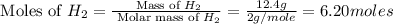
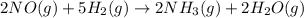
 react with 5 mole of
react with 5 mole of 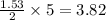 moles of
moles of