
Chemistry, 09.11.2019 05:31, brooklynpage3930
The heats of combustion of ethane (c2h6) and butane (c4h10) are 52 kj/g and 49 kj/g, respectively. we need to produce 1.000 x 103 kj heat by burning one of the fuels. which fuel will emit the least amount of co2? 1. calculate the number of grams needed of each fuel: 2. calculate the number of moles of each fuel: 3. write down the balanced chemical equation for the combustion of the fuels: 4. calculate the number of moles of co2 produced by burning each fuel to produce 1.000 x 103 kj. which fuel will emit the least amount of co2?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, mpchop
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 02:00, bernicewhite156
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Do you know the correct answer?
The heats of combustion of ethane (c2h6) and butane (c4h10) are 52 kj/g and 49 kj/g, respectively. w...
Questions in other subjects:

Mathematics, 14.12.2020 20:30


Chemistry, 14.12.2020 20:30

History, 14.12.2020 20:30

Mathematics, 14.12.2020 20:30


Mathematics, 14.12.2020 20:30

English, 14.12.2020 20:30



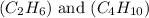 are 19.23 g and 20.41 g respectively.
are 19.23 g and 20.41 g respectively.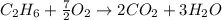

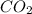 produced by burning each fuel is 1.28 mole and 1.41 mole respectively.
produced by burning each fuel is 1.28 mole and 1.41 mole respectively.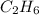

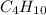 = 1 g
= 1 g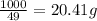

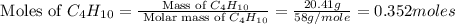
 moles of
moles of  moles of
moles of 



