
Chemistry, 09.11.2019 05:31, lexjenae8519
The volume of a gas held at constant temperature varies indirectly as the pressure of the gas. if the volume of a gas is 1200 cubic centimeters when the pressure is 400 millimeters of mercury, what is the volume, v, when the pressure is 500 millimeters of mercury?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, melikefood01
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1


Chemistry, 23.06.2019 02:00, Paytonsmommy09
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2

Do you know the correct answer?
The volume of a gas held at constant temperature varies indirectly as the pressure of the gas. if th...
Questions in other subjects:

Mathematics, 23.06.2019 14:00

Mathematics, 23.06.2019 14:00

Mathematics, 23.06.2019 14:00

Geography, 23.06.2019 14:00

English, 23.06.2019 14:00



Computers and Technology, 23.06.2019 14:00



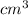

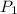 and
and  are initial and final pressure of the gas respectively.
are initial and final pressure of the gas respectively.  and
and 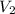 are initial and final volume of the gas respectively.
are initial and final volume of the gas respectively.  and
and  are initial and final temperature of the gas in kelvin respectively.
are initial and final temperature of the gas in kelvin respectively.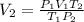
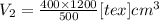 =960
=960 




