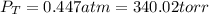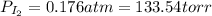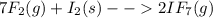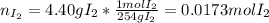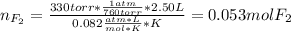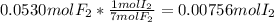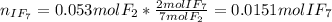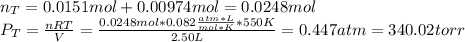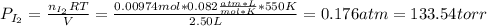Chemistry, 09.11.2019 05:31, jimennacastillo15
When gaseous f2 and solid i2 are heated to high temperatures, the i2 sublimes and gaseous iodine heptafluoride forms.3.30 × 102 torr of f2 and 4.40 g of solid i2 are put into a 2.50 l container at 2.50 × 102 k and the container is heated to 5.50 × 10^2 k.
(a) what is the final pressure?
ptotal =
(b) what is the partial pressure of i2 gas?
pi2 = i2

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, paynedeforest2596
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 12:30, kingbot350
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1
Do you know the correct answer?
When gaseous f2 and solid i2 are heated to high temperatures, the i2 sublimes and gaseous iodine hep...
Questions in other subjects:

Mathematics, 04.01.2022 14:00



Mathematics, 04.01.2022 14:00