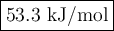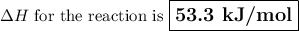Chemistry, 08.11.2019 22:31, debrielcalderon
Use hess's law to calculate the enthalpy change for the reaction: 3c(s) + 3h2(g) → c3h6(g) given the following thermochemical equations: 2c3h6(g) + 9o2(g) → 6co2(g) + 6h2o(l) δh = -4182.6 kj/mol c(s) + o2(g) → co2(g) δh = -393.51 kj/mol h2(g) + ½o2(g) → h2o(l) δh = -285.83 kj/mol

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, Chente379
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 23.06.2019 00:20, HernanJe6
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1


Chemistry, 23.06.2019 10:00, Sariyahhall1
Two moles of potassium chloride and three moles of oxygen are produced from the decomposition of two moles of potassium chlorate, kcos3(s). write the balanced equation. how many moles of oxygen are produced from 12 moles of potassium chlorate
Answers: 1
Do you know the correct answer?
Use hess's law to calculate the enthalpy change for the reaction: 3c(s) + 3h2(g) → c3h6(g) given th...
Questions in other subjects:



History, 20.07.2019 01:00





Social Studies, 20.07.2019 01:00

History, 20.07.2019 01:00

Biology, 20.07.2019 01:00