For the reaction:
2n2o5(g) → 4no2(g) + o2(g) the rate law is: (δ[o2]/δt) = k[n2o5] at 300 k...

Chemistry, 08.11.2019 22:31, alexciamartinez05
For the reaction:
2n2o5(g) → 4no2(g) + o2(g) the rate law is: (δ[o2]/δt) = k[n2o5] at 300 k, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kj/mol. what is the half-life at 310 k? (hint: use rate law expression to determine the reaction order → solve for k1 at 300 k using the corresponding half-life expression → use two-point arrhenius equation to solve for k2 at 310 k → use the half-life expression again to solve for half-life at 310 k)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 18:30, chinadoll24
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2


Do you know the correct answer?
Questions in other subjects:


English, 01.07.2019 05:00

Mathematics, 01.07.2019 05:00

Mathematics, 01.07.2019 05:00

Mathematics, 01.07.2019 05:00

Social Studies, 01.07.2019 05:00

Mathematics, 01.07.2019 05:00



Mathematics, 01.07.2019 05:00



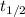 is the half-life
is the half-life is the rate constant
is the rate constant