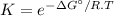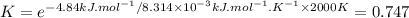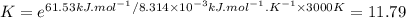Chemistry, 08.11.2019 05:31, ekerns2000paa19x
The decomposition of a generic diatomic element in its standard state is represented by the equation 12x2(g)⟶x(g) assume that the standard molar gibbs energy of formation of x(g) is 4.84 kj·mol−1 at 2000 . k and −61.53 kj·mol−1 at 3000 . k. determine the value of the thermodynamic equilibrium constant, k , at each temperature. at 2000 . k, δ=4.84 kj·mol−1 . what is k at that temperature?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 12:00, macylen3900
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2
Do you know the correct answer?
The decomposition of a generic diatomic element in its standard state is represented by the equation...
Questions in other subjects:

Mathematics, 22.05.2021 23:10

Mathematics, 22.05.2021 23:10


Biology, 22.05.2021 23:10





Mathematics, 22.05.2021 23:10