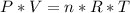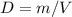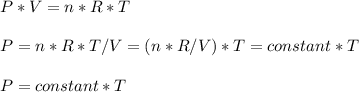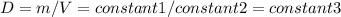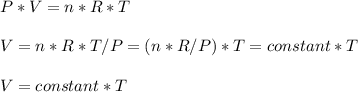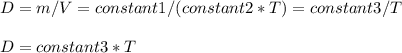Chemistry, 08.11.2019 03:31, kamkam5791
Consider two different containers, each filled with of . one of the containers is rigid and has constant volume. the other container is flexible (like a balloon) and is capable of changing its volume to keep the external pressure and internal pressure equal to each other. if you raise the temperature in both containers, what happens to the pressure and density of the gas inside each container? assume a constant external pressure.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:40, allyyzz
Astudent is given a sample of a blue copper sulfate hydrate. he weighs the sample in a dry covered porcelain crucible and got a mass of 23.875 g for the crucible, lid, and sample. the mass of the empty crucible and lid was found earlier to be 22.652 g. he then heats the crucible to expel the water of hydration, keeping the crucible at red heat for 10 minutes with the lid slightly ajar. on colling, he finds the mass of crucible, lid, and contents to be 23.403 g. the sample was changed in the process to very light clue anhydrous cuso4. if there are again 100.0 g of hydrate, how many grams of cuso4 are in it? how many moles of cuso4? (hint: molar mass of cuso4 = 159.6 g / mole. what per cent of the hydrate is cuso4? you may convert the mass of cuso4 to moles.)
Answers: 3

Chemistry, 22.06.2019 04:00, clairebear66
What three natural resources are found in the great lakes region
Answers: 2


Chemistry, 22.06.2019 13:00, monkeyrose1999
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3
Do you know the correct answer?
Consider two different containers, each filled with of . one of the containers is rigid and has cons...
Questions in other subjects:


Mathematics, 01.02.2021 19:30


English, 01.02.2021 19:30


Mathematics, 01.02.2021 19:30


English, 01.02.2021 19:30


Mathematics, 01.02.2021 19:30