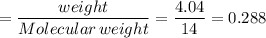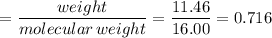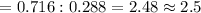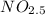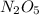Chemistry, 08.11.2019 02:31, kkeith121p6ujlt
If 4.04g of n combine with 11.46g o to produce a compound with a molar mass of 108.0 g/mol, what is the molecular formula of this compound?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:30, jaydenbrock
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 23.06.2019 00:00, alisonsolis155
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3

Do you know the correct answer?
If 4.04g of n combine with 11.46g o to produce a compound with a molar mass of 108.0 g/mol, what is...
Questions in other subjects:



Mathematics, 31.03.2020 18:00

Mathematics, 31.03.2020 18:00



English, 31.03.2020 18:00


Mathematics, 31.03.2020 18:00

Mathematics, 31.03.2020 18:00