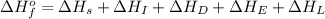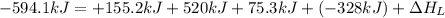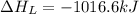Chemistry, 07.11.2019 22:31, kraigstlistt
The process of forming an ionic salt from its constituent metallic and nonmetallic elements is called the born-haber cycle, which is a series of thermochemical processes, each with a δh, that add up (think of hess’s law) to complete a 5 step process for the formation of the salt. given the following data, calculate the lattice energy per mole of lif(s) formed. li(s) → li(g) δh°

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, juliannxkim
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Do you know the correct answer?
The process of forming an ionic salt from its constituent metallic and nonmetallic elements is calle...
Questions in other subjects:


History, 17.07.2020 08:01

Physics, 17.07.2020 08:01





Mathematics, 17.07.2020 08:01


Mathematics, 17.07.2020 08:01


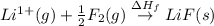
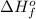 = enthalpy of formation of lithium fluoride = -594.1 kJ
= enthalpy of formation of lithium fluoride = -594.1 kJ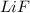 :
: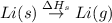
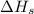 = sublimation energy of lithium = +155.2 kJ
= sublimation energy of lithium = +155.2 kJ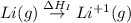
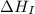 = ionization energy of lithium = +520 kJ
= ionization energy of lithium = +520 kJ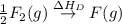
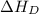 = dissociation energy of fluorine = +75.3 kJ
= dissociation energy of fluorine = +75.3 kJ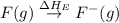
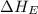 = electron affinity energy of fluorine = -328 kJ
= electron affinity energy of fluorine = -328 kJ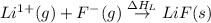
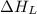 = lattice energy of lithium fluoride = ?
= lattice energy of lithium fluoride = ?