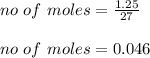Assuming you start with 1.25 g of pure aluminum, calculate the following:
1) the amount of po...


Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, natalie857123
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 07:20, mathman783
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 09:40, cheesecake1919
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Do you know the correct answer?
Questions in other subjects:

Mathematics, 15.04.2021 20:20




Mathematics, 15.04.2021 20:20