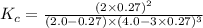Chemistry, 07.11.2019 06:31, danielobanoyen
Initially 2.0 moles of n2(g) and 4.0 moles of h2(g) were added to a 1.0-liter container and the following reaction then occurred: 3h2(g) + n2(g) 2nh3(g) the equilibrium concentration of nh3(g) = 0.55 moles/liter at 700.°c. what is the value for k at 700.°c for the formation of ammonia?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:00, aliciaa101
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 23.06.2019 01:00, Johnson926
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2

Chemistry, 23.06.2019 05:20, cjking2320
Explain how global warming could have affected yellowstone frog and salamander habitat's, resulting in changes in the populations of these species
Answers: 2

Do you know the correct answer?
Initially 2.0 moles of n2(g) and 4.0 moles of h2(g) were added to a 1.0-liter container and the foll...
Questions in other subjects:

Mathematics, 07.08.2019 00:10

History, 07.08.2019 00:10


Mathematics, 07.08.2019 00:10




Social Studies, 07.08.2019 00:10

Mathematics, 07.08.2019 00:10


 for the reaction is,
for the reaction is, 
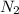 = 2.0 mol
= 2.0 mol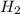 = 4.0 mol
= 4.0 mol at equilibrium = 0.55 mol/L = 0.55 M
at equilibrium = 0.55 mol/L = 0.55 M .
.
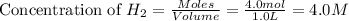

![K_c=\frac{[NH_3]^2}{[N_2][H_2]^3}](/tpl/images/0363/4597/c3aa0.png)
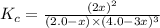 .......(1)
.......(1)