
A0.05270.0527 g piece of metal containing an unknown amount of silver is dissolved to form 25.00 ml of solution. to the solution, 35.435.4 ml of 0.02190.0219 m kcl kcl is added, resulting in the complete precipitation of the silver ash agcl agcl . the excess cl−cl− added to the solution is then back titrated with a standard 0.02190.0219 m agno3 agno3 solution. a total of 14.3314.33 ml of the standard nano3 agno3 solution is required to reach the silver chromate end point in the mohr method. calculate the percent silver in the piece of metal.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, micvar9646
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3


Chemistry, 22.06.2019 15:00, levelebeasley1
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
Do you know the correct answer?
A0.05270.0527 g piece of metal containing an unknown amount of silver is dissolved to form 25.00 ml...
Questions in other subjects:







Mathematics, 15.01.2020 21:31




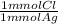 = 0.314 mmol Cl⁻
= 0.314 mmol Cl⁻



