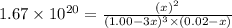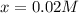Chemistry, 07.11.2019 06:31, winterblanco
0.0200 m fe3+ is initially mixed with 1.00 m oxalate ion, c2o42-, and they react according to the equation: fe3+(aq) + 3 c2o42-(aq) ⇄ [fe(c2o4)3]3-(aq) kc = 1.67 × 1020 what is the concentration of fe3+(aq) when equilibrium is reached? 1.67 × 1020 m 8.35 × 10-19 m 6.9a × 1021 m 1.44 × 10-22 m 0.980 am 0.940 m 0.0100 atm

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2


Chemistry, 22.06.2019 21:00, alwaysneedhelp84
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
Do you know the correct answer?
0.0200 m fe3+ is initially mixed with 1.00 m oxalate ion, c2o42-, and they react according to the eq...
Questions in other subjects:

Mathematics, 23.07.2019 22:20




Biology, 23.07.2019 22:20




Biology, 23.07.2019 22:20


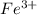 at equilibrium is 0 M.
at equilibrium is 0 M. = 1.00 M
= 1.00 M![Fe^{3+}(aq)+3C_2O_4^{2-}(aq)\rightleftharpoons [Fe(C_2O_4)_3]^{3-}(aq)](/tpl/images/0363/4634/1f551.png)
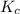 will be,
will be,![K_c=\frac{[[Fe(C_2O_4)_3]^{3-}]}{[C_2O_4^{2-}]^3[Fe^{3+}]}](/tpl/images/0363/4634/137fa.png)