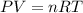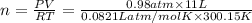Chemistry, 07.11.2019 05:31, mckleinrivero
Amaterials scientist has created an alloy containing aluminum, copper, and zinc, and wants to determine the percent composition of the alloy. the scientist takes a 13.039 g sample of the alloy and reacts it with concentrated hcl . the reaction converts all of the aluminum and zinc in the alloy to aluminum chloride and zinc chloride in addition to producing hydrogen gas. the copper does not react with the hcl . upon completion of the reaction, a total of 11 l of hydrogen gas was collected at a pressure of 744 torr and a temperature of 27.0 °c . additionally, 2.761 g of unreacted copper is recovered. calculate the mass of hydrogen gas formed from the reaction.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:40, georgehall3027
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 02:00, rosie20052019
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 03:30, electrofy456
What diagram shows the ionic compound of magnesium oxide
Answers: 2

Chemistry, 22.06.2019 06:30, backup5485
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1
Do you know the correct answer?
Amaterials scientist has created an alloy containing aluminum, copper, and zinc, and wants to determ...
Questions in other subjects: