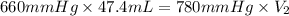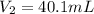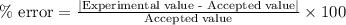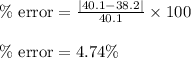Chemistry, 07.11.2019 05:31, annagracedavis2002
Boyle's law-effect of pressure at constant temperature imagine that you performed this experiment as described in your manual. in doing so, your first pressure reading was 660 mmhg, and your second presure reading was 780 mmig your first volume was 474 ml what would the percent error be if your measured second volume was 38.2 ml ? 4.76 x incorrect, one attempt remaining

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, alexusnicole817
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 09:50, revlonknox6
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2


Chemistry, 23.06.2019 01:30, oliviacolaizzi
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1
Do you know the correct answer?
Boyle's law-effect of pressure at constant temperature imagine that you performed this experiment as...
Questions in other subjects:


Mathematics, 21.10.2020 20:01




Mathematics, 21.10.2020 20:01



Mathematics, 21.10.2020 20:01


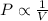
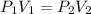
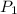 = first pressure = 660 mmHg
= first pressure = 660 mmHg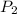 = second pressure = 780 mmHg
= second pressure = 780 mmHg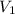 = first volume = 47.4 mL
= first volume = 47.4 mL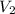 = second volume = ?
= second volume = ?