
During a spontaneous chemical reaction, it is found that δssys is less than 0. this means that group of answer choices δssurr is less than 0 and its magnitude is less than δssys. δssurr is less than 0 and its magnitude is greater than δssys. δssurr is greater than 0 and its magnitude is less than δssys. δssurr is greater than 0 and its magnitude is greater than δssys. an error has been made, as ssys is greater than 0 by necessity for a spontaneous process.

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 04:00, fantasticratz2
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 23:30, emmalado45
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1
Do you know the correct answer?
During a spontaneous chemical reaction, it is found that δssys is less than 0. this means that group...
Questions in other subjects:

History, 13.10.2019 08:10



English, 13.10.2019 08:10




Mathematics, 13.10.2019 08:20


Mathematics, 13.10.2019 08:20


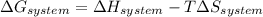
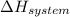 is the enthalpy change and
is the enthalpy change and 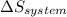 is the entropy change of the system.
is the entropy change of the system.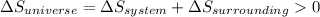
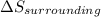 should be greater than 0 or positive. Also,
should be greater than 0 or positive. Also, 



