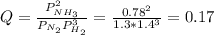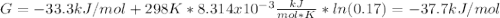In the haber process, ammonia is synthesized from nitrogen and hydrogen: n2(g)+3h2(g)→2nh3(g) δg∘ at 298k for this reaction is −33.3kj/mol. the value of δg at 298k for a reaction mixture that consists of 1.3atmn2, 1.4atmh2, and 0.78atmnh3 is kj/mol. in the haber process, ammonia is synthesized from nitrogen and hydrogen: at for this reaction is . the value of at for a reaction mixture that consists of , , and is . −4.42×103 −76.6 −37.7 −5.7 −2.13 × 103

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:00, jazmine8194
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1


Chemistry, 23.06.2019 03:30, alvfran1041
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1
Do you know the correct answer?
In the haber process, ammonia is synthesized from nitrogen and hydrogen: n2(g)+3h2(g)→2nh3(g) δg∘ a...
Questions in other subjects:



Mathematics, 22.11.2020 03:20


Mathematics, 22.11.2020 03:20

Mathematics, 22.11.2020 03:20

Geography, 22.11.2020 03:20


Mathematics, 22.11.2020 03:20

Mathematics, 22.11.2020 03:20


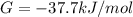
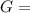 Δ
Δ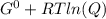
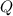 is computed via the law of mass action:
is computed via the law of mass action: