
Chemistry, 07.11.2019 01:31, kaitlynmeats
Consider the titration of 20.00 ml of 0.754 m sodium benzoate with a solution of 0.525 m nitric acid. a. calculate the equivalence volume in ml. b. calculate the ph at the equivalence point. c. calculate the ph of the solution after addition of 16.20 ml nitric acid. d. calculate the ph of the solution after addition of 39.82 ml nitric acid.

Answers: 3
Similar questions

Chemistry, 30.08.2019 18:20, hetdashadia123
Answers: 1

Chemistry, 10.10.2019 05:50, jjelzy
Answers: 1

Chemistry, 24.10.2019 03:50, ziar7176
Answers: 1

Chemistry, 20.11.2019 21:31, dixonmckenzie1429
Answers: 1
Do you know the correct answer?
Consider the titration of 20.00 ml of 0.754 m sodium benzoate with a solution of 0.525 m nitric acid...
Questions in other subjects:











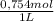 = 0,01508mol of NaBz
= 0,01508mol of NaBz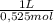 = 0,0287L = 28,7 mL
= 0,0287L = 28,7 mL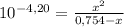
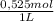 = 5,838x10⁻³moles of H⁺. The volume is 20,0mL + 39,82mL = 59,82mL ≡ 0,05982L. Thus, [H⁺] is:
= 5,838x10⁻³moles of H⁺. The volume is 20,0mL + 39,82mL = 59,82mL ≡ 0,05982L. Thus, [H⁺] is:



