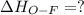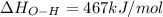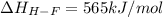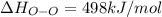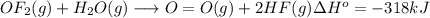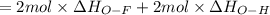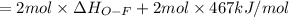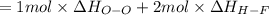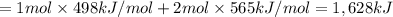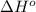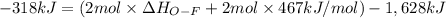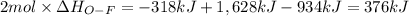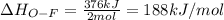Chemistry, 07.11.2019 01:31, jenlicavoli
Oxygen difluoride is an unstable molecule that reacts readily with water. calculate the bond energy of the o–f bond using the standard enthalpy of reaction and the bond energy data provided. just enter a number (no units). of2(g) + h2o(g) \longrightarrow⟶ o=o(g) + 2hf(g) \deltaδh° = –318 kj bond: o–h o=o h–f bond energy (kj/mol): 467 498 565

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, jescanarias22
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1


Chemistry, 22.06.2019 04:40, shanicar33500
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1
Do you know the correct answer?
Oxygen difluoride is an unstable molecule that reacts readily with water. calculate the bond energy...
Questions in other subjects:



Mathematics, 25.11.2021 06:40

Mathematics, 25.11.2021 06:40



Social Studies, 25.11.2021 06:40