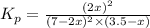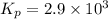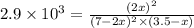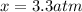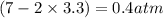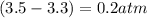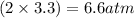Chemistry, 06.11.2019 04:31, PlsHelpMeh3401
Calculate the pressures of no, cl2, and nocl in an equilibrium mixture produced by the reaction of a starting mixture with 7.0 atm no and 3.5 atm cl2. (hint: kp is relatively large; assume the reaction goes to completion then comes back to equilibrium.)
2 no(g) + cl2(g) --> 2 nocl(g)kp = 2.9 ✕ 103 at 149°c

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 03:20, coollid876
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3

Chemistry, 23.06.2019 04:20, milkshakegrande101
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1
Do you know the correct answer?
Calculate the pressures of no, cl2, and nocl in an equilibrium mixture produced by the reaction of a...
Questions in other subjects:

English, 17.11.2020 20:20


Mathematics, 17.11.2020 20:20



Mathematics, 17.11.2020 20:20

English, 17.11.2020 20:20



Biology, 17.11.2020 20:20


 ,
, 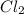 , and
, and 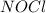 in an equilibrium mixture are 0.4 atm , 0.2 atm and 6.6 atm respectively.
in an equilibrium mixture are 0.4 atm , 0.2 atm and 6.6 atm respectively.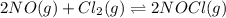
![K_p=\frac{[NOCl]^2}{[NO]^2[Cl_2]}](/tpl/images/0361/4899/09f8c.png)