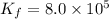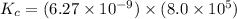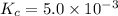Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:10, Tyrant4life
Which of these is the result of scientific research and not engineering? a. a new shoe design that features air cushioning for more comfort and protection b. the creation of glass with uv protection. c. a conclusion about diet commonalities among diabetics. d. the development of a smaller, more compact missile.
Answers: 1

Chemistry, 21.06.2019 20:10, irene4523
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1

Chemistry, 22.06.2019 17:00, destinyycooper
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 20:30, demarcuswiseman
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
Do you know the correct answer?
Calculate the value of the equilibrium constant, kc, for the reaction cubr(s) + br−(aq) ↽⇀ cubr−2(aq...
Questions in other subjects:

English, 10.10.2021 05:50





Mathematics, 10.10.2021 05:50

Chemistry, 10.10.2021 05:50


Mathematics, 10.10.2021 05:50


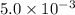
![CuBr(s)+Br^-(aq)\rightleftharpoons [CuBr_2]^-(aq)](/tpl/images/0361/4240/23bce.png)
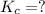

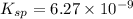
![Cu^+(aq)+2Br^-(aq)\rightleftharpoons [CuBr_2]^-(aq)](/tpl/images/0361/4240/ae2b9.png)