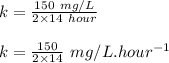Engineers do a treatment study on an industrial waste containing chemical x. in a batch reactor, the concentration of chemical x drops from 150 mg/l to 75 mg/l in 14 hours.
(a) what is the half-life?
(b) what is the decay constant if the reaction is first order?
(c) what is the decay constant if the reaction is zero order?
(d) how would you determine whether the reaction is zero or first order? if you had a set of concentration

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:20, alexis3060
How do you know when a chemical reaction has occurred
Answers: 1

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
Do you know the correct answer?
Engineers do a treatment study on an industrial waste containing chemical x. in a batch reactor, the...
Questions in other subjects:


History, 31.01.2020 02:48


Biology, 31.01.2020 02:48


History, 31.01.2020 02:48



Mathematics, 31.01.2020 02:48

World Languages, 31.01.2020 02:48


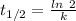
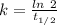
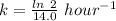
![t_{1/2}=\frac{[A]_0}{2k}](/tpl/images/0361/2925/15a8b.png)
![[A]_0](/tpl/images/0361/2925/7075c.png) is the initial concentration = 150 mg/L
is the initial concentration = 150 mg/L