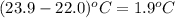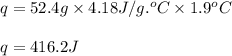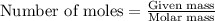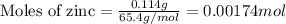Zinc metal reacts with hydrochloric acid according to this balanced equation. zn(s) 2hcl(aq)→zncl2(aq) h2(g) when 0.114 g of zn(s) is combined with enough hcl to make 52.4 ml of solution in a coffee-cup calorimeter, all of the zinc reacts, raising the temperature of the solution from 22.0 ∘c to 23.9 ∘c.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, yyyyyy290
Why are gamma rays not affected by a magnet as they pass over it? gamma rays are composed of only energy. gamma rays do not have enough mass to be affected. gamma rays do not have the right electrical charge to be affected. gamma rays move too fast for anything to affect their pathway.
Answers: 1


Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1
Do you know the correct answer?
Zinc metal reacts with hydrochloric acid according to this balanced equation. zn(s) 2hcl(aq)→zncl2(a...
Questions in other subjects:

Mathematics, 02.02.2020 23:50

Chemistry, 02.02.2020 23:50

English, 02.02.2020 23:50



History, 02.02.2020 23:50


Social Studies, 02.02.2020 23:50



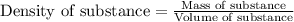
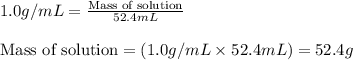
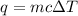
 = change in temperature =
= change in temperature =