
Chemistry, 06.11.2019 02:31, kimbely7704
The molar heat capacity of ethane is represented in the temperature range 298 k to 400 k by the empirical expression cp, m in j k1 mol 14.73 + (0.1272 t in k). the corresponding expressions for c(e) and h2(g) are given in the back of the atkins textbook. calculate the standard enthalpy of formation of ethane at 373 k from its value at 298 k, in kj mol

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, JuniperGalaxy
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 15:00, alanmarcus22
What does the symbol (–hfus) indicate in a phase change?
Answers: 1

Chemistry, 22.06.2019 23:00, catdog5225
What is formed when amino acids form long chains or polymerize
Answers: 1

Chemistry, 22.06.2019 23:10, RealStephani
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(s o4)2·7h2omgso4·7h2o
Answers: 1
Do you know the correct answer?
The molar heat capacity of ethane is represented in the temperature range 298 k to 400 k by the empi...
Questions in other subjects:

Biology, 20.11.2019 14:31






History, 20.11.2019 14:31


Health, 20.11.2019 14:31

Geography, 20.11.2019 14:31


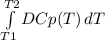
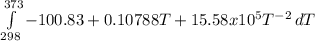 = -3796.48 J/mol = -3.80 kJ/mol (solved by a graphic calculator)
= -3796.48 J/mol = -3.80 kJ/mol (solved by a graphic calculator)



