
Avogadro constant is 6.02 1023 (the number of atoms or molecules per mole). what how much charge is there in 1 mole of electrons? how much charge is there in 1 mole of hydrogen ions (h+). remember that hydrogen consists of one electron and one proton, so hydrogen ions are just protons.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, daniel9299
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 06:00, applejulianamoreno
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 07:00, haydjanggg6578
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 09:00, 2024cynthiatercero
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3
Do you know the correct answer?
Avogadro constant is 6.02 1023 (the number of atoms or molecules per mole). what how much charge is...
Questions in other subjects:

History, 25.04.2021 22:40

History, 25.04.2021 22:40

History, 25.04.2021 22:40



Biology, 25.04.2021 22:50

Business, 25.04.2021 22:50

Mathematics, 25.04.2021 22:50

Arts, 25.04.2021 22:50


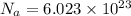
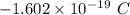
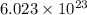 electrons =
electrons = 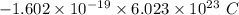 = -96488.46 C
= -96488.46 C



