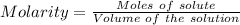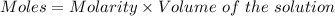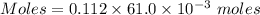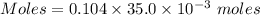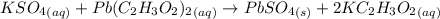A61.0ml sample of a 0.112m potassium sulfate solution is mixed with 35.0ml of a 0.104m lead(ii) acetate solution and the following precipitation reaction occurs:
k2so4(aq)+pb(c2h3o2)2(aq)? 2kc2h3o2(aq)+pbso4(s)
the solid pbso4 is collected, dried, and found to have a mass of 0.997g .
determine the limiting reactant, the theoretical yield, and the percent yield.
part a.
identify the limiting reactant.
kc2h3o2
pb(c2h3o2)2
k2so4
pbso4
part b.
determine the theoretical yield.
mass of pbso4 =
part c.
determine the percent yield=

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, zaehairston78531
What is the nature of the ca-cl bond in a molecule of calcium chloride (cacl2) if the electronegativity value of calcium is 1.0 and that of chlorine is 3.16?
Answers: 1

Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2

Chemistry, 22.06.2019 20:40, oddoneshenchman
Why do lunar and solar eclipse not happen every month
Answers: 2
Do you know the correct answer?
A61.0ml sample of a 0.112m potassium sulfate solution is mixed with 35.0ml of a 0.104m lead(ii) acet...
Questions in other subjects:



Mathematics, 16.11.2020 18:10


English, 16.11.2020 18:10




Mathematics, 16.11.2020 18:10

SAT, 16.11.2020 18:10