
Chemistry, 05.11.2019 05:31, sainijasdeep27
Three glass bulbs, joined by closed stopcocks, have the following volumes and initial pressures of the specified gases.
bulb a: 200. ml of kr(g) at 190. torr
bulb b: 400. ml of h2s(g) at 1.00 atm
bulb c: 1.00 l of n2(g) at 75.994 kpa
after both stopcocks are opened and the gases allowed to diffuse throughout, what will be the ultimate total pressure?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:20, sindy35111
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l. s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3


Chemistry, 22.06.2019 19:30, gracieisweird12
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 23.06.2019 01:00, liamgreene90
The time that is taken by neptune once around the sun is called
Answers: 1
Do you know the correct answer?
Three glass bulbs, joined by closed stopcocks, have the following volumes and initial pressures of t...
Questions in other subjects:

History, 05.02.2021 16:50


History, 05.02.2021 16:50




Biology, 05.02.2021 16:50


Mathematics, 05.02.2021 16:50

Health, 05.02.2021 16:50




 P (atm)
P (atm)
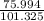 atm
atm





