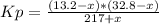An equilibrium mixture of pcl₅(g), pcl₃(g), and cl₂(g) has partial pressures of 217.0 torr, 13.2 torr, and 13.2 torr, respectively. a quantity of cl₂(g) is injected into the mixture, and the total pressure jumps to 263.0 torr. the appropriate chemical equation is pcl₃(g)+cl₂(g)↽−−⇀pcl₅(g) calculate the new partial pressures after equilibrium is reestablished.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:40, gonzaleze18
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 17:30, tiffanyhmptn
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1


Chemistry, 23.06.2019 02:00, hermesrobles
Which would freeze at a higher temperature: the great salt lake or lake tahoe? a. lake tahoe would freeze at a higher temperature. b. the great salt lake would freeze at a higher temperature. c. both lakes would freeze at the same temperature.
Answers: 2
Do you know the correct answer?
An equilibrium mixture of pcl₅(g), pcl₃(g), and cl₂(g) has partial pressures of 217.0 torr, 13.2 tor...
Questions in other subjects:

Social Studies, 09.03.2021 01:00



Mathematics, 09.03.2021 01:00


Mathematics, 09.03.2021 01:00





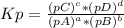 , where pX is the partial pressure of X.
, where pX is the partial pressure of X.