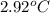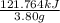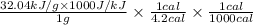Chemistry, 05.11.2019 04:31, humphreybrittany42
Aresearcher studying the nutritional value of a new candy places a 3.80 g3.80 g sample of the candy inside a bomb calorimeter and combusts it in excess oxygen. the observed temperature increase is 2.92 ∘c.2.92 ∘c. if the heat capacity of the calorimeter is 41.70 kj⋅k−1,41.70 kj⋅k−1, how many nutritional calories are there per gram of the candy?

Answers: 3
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 08:00, george27212
What is the temperature in kelvin of a gas if it is allowed to expand from 1.50 l to 4.50 l? the initial temperature is 10.0°c and pressure is constant throughout the change. which equation should you use? t2= v2/v1 t1 what is the final temperature? ⇒ 849 k these are the answers.
Answers: 1
Do you know the correct answer?
Aresearcher studying the nutritional value of a new candy places a 3.80 g3.80 g sample of the candy...
Questions in other subjects:

History, 18.04.2021 06:00


Mathematics, 18.04.2021 06:00


Mathematics, 18.04.2021 06:00

Computers and Technology, 18.04.2021 06:00

Chemistry, 18.04.2021 06:00