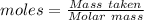Compounds of boron and hydrogen are remarkable for their unusual bonding and also for their reactivity. with the more reactive halogens, for example, diborane (b2h6) forms trihalides even at low temperatures: b2h6(g) + 6 cl2(g) → 2 bcl3(g) + 6 hcl(g) δ hrxn = −755.4 kj how much heat is released when 4.465 kg of diborane reacts? (give your answer in scientific notation.)

Answers: 2
Similar questions


Chemistry, 26.06.2019 11:00, maddie3354
Answers: 1

Chemistry, 15.07.2019 16:10, gizmo50245
Answers: 1
Do you know the correct answer?
Compounds of boron and hydrogen are remarkable for their unusual bonding and also for their reactivi...
Questions in other subjects:









History, 20.09.2020 04:01



 :-
:-