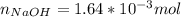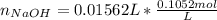Chemistry, 05.11.2019 02:31, powellmom5
Atablet of pain be gone aspirin, which had a mass of 1.213 g, was pulverized and 1.159 g were dissolved in 10.0 ml of ethyl alcohol and 25.0 ml of di water. the titration of this solution with 0.1052 m naoh required 15.62 ml to reach the phenolphthalein endpoint. determine the moles of naoh that reacted with the acetylsalicylic acid.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, cynthiagutierrez65
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1

Chemistry, 22.06.2019 20:30, camerondillonn
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 22.06.2019 23:00, DESI111609
What is the average rate of the reaction between 10 and 20 s?
Answers: 1

Chemistry, 23.06.2019 01:00, jazzy200232
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
Do you know the correct answer?
Atablet of pain be gone aspirin, which had a mass of 1.213 g, was pulverized and 1.159 g were dissol...
Questions in other subjects:

Mathematics, 02.03.2021 21:40

Spanish, 02.03.2021 21:40





Mathematics, 02.03.2021 21:40

Mathematics, 02.03.2021 21:40