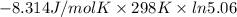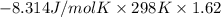Chemistry, 05.11.2019 00:31, jessemartinez1
For the reaction a(g) + 2 b(g) ↔ c(g) the initial partial pressures of gases a, b, and c are all 0.109 atm. once equilibrium has been established, it is found that pc = 0.047 atm. what is δg° for this reaction (in kj/mol) at 25°c?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, ayoismeisjjjjuan
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 07:30, veronica25681
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 09:00, Aminton737
Plz mark brainliest 30 points1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s.2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Do you know the correct answer?
For the reaction a(g) + 2 b(g) ↔ c(g) the initial partial pressures of gases a, b, and c are all 0.1...
Questions in other subjects:





Mathematics, 28.03.2020 21:57

Mathematics, 28.03.2020 21:57

Biology, 28.03.2020 21:57

Social Studies, 28.03.2020 21:57

Mathematics, 28.03.2020 21:57


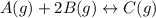
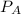 = 0.109 atm,
= 0.109 atm, 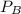 = 0.109 atm,
= 0.109 atm,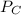 = 0.109 atm
= 0.109 atm![[0.109 + (2 \times 0.062)]](/tpl/images/0359/5284/f1aca.png) atm
atm 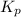 as follows.
as follows.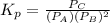
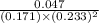
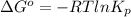
 as follows.
as follows.