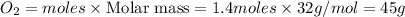Question 51 pts aluminum reacts with oxygen to produce aluminum oxide which can be used as an adsorbent, desiccant, or catalyst for organic reactions. a mixture of 82.49 g of aluminum and 117.65 g of oxygen is allowed to react. what is the mass of the excess reactant present in the vessel when the reaction is complete? report your answer to the appropriate number of significant figures.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, rscott2649
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 08:30, aydenmasonc
Which statement describes james chadwick’s discovery.
Answers: 2

Do you know the correct answer?
Question 51 pts aluminum reacts with oxygen to produce aluminum oxide which can be used as an adsorb...
Questions in other subjects:


Mathematics, 24.03.2020 00:53


History, 24.03.2020 00:53

Mathematics, 24.03.2020 00:53





Mathematics, 24.03.2020 00:53


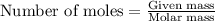
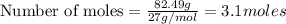
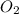
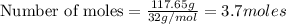
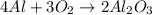
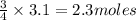 of
of