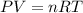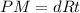Chemistry, 05.11.2019 00:31, Imamdiallo18
Assuming ideal behavior, one mole krypton gas is released into flexible container at stp. what is the density of krypton gas in this container at stp? at stp one mole of any gas occupies 22.4 l. (r = 0.08206 l • atm/k • mol) atomic mass of krypton is 83.8 amu. select one: a. 0.0561 g/l b. 0.0176 g/l c. 3.74 g/l d. 181 g/l e. 1.78 g/l

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, sethjohnson386pbnm3x
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 21:30, imalexiscv
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1

Chemistry, 23.06.2019 07:30, lucas2020197
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 2
Do you know the correct answer?
Assuming ideal behavior, one mole krypton gas is released into flexible container at stp. what is th...
Questions in other subjects:

Mathematics, 26.12.2020 21:30

World Languages, 26.12.2020 21:30

Mathematics, 26.12.2020 21:30

Chemistry, 26.12.2020 21:30


English, 26.12.2020 21:30

Mathematics, 26.12.2020 21:30

Physics, 26.12.2020 21:30


Physics, 26.12.2020 21:30