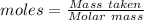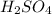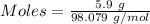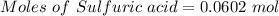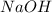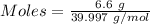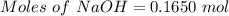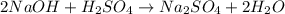Chemistry, 05.11.2019 00:31, safiyabrowne7286
Aqueous sulfuric acid (h2so4) reacts with solid sodium hydroxide (naoh) to produce aqeous sodium sulfate (na2so4) and liquid water (h2o). what is the theoretical yield of water formed from the reaction of 5.9 g of sulfuric acid and 6.6 g of sodium hydroxide?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:00, ahmedeldyame
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1

Chemistry, 23.06.2019 02:00, raulflores01
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1

Chemistry, 23.06.2019 04:31, mdarter
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2
Do you know the correct answer?
Aqueous sulfuric acid (h2so4) reacts with solid sodium hydroxide (naoh) to produce aqeous sodium sul...
Questions in other subjects:


Biology, 07.04.2021 21:20


Mathematics, 07.04.2021 21:20

Social Studies, 07.04.2021 21:20


Mathematics, 07.04.2021 21:20

Mathematics, 07.04.2021 21:20

Biology, 07.04.2021 21:20

Mathematics, 07.04.2021 21:20