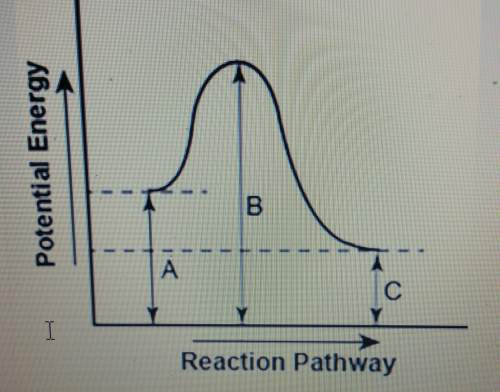
The diagram shows the potential energy changes for a reaction pathway.
1. does the diag...

The diagram shows the potential energy changes for a reaction pathway.
1. does the diagram illustrate an endothermic or exothermic reaction? give reasons to support your answer?
2. describe how you can determine the total change in enthalpy and activation energy and if each is positive or negative.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, Sarahinator04
0.09 moles of sodium sulfate in 12 ml of solution
Answers: 3


Chemistry, 23.06.2019 07:00, jstyopin
In order for a high temperature boiler or steam engine to produce superheated water, or steam: the heat source must be greater than 100°c the water must be permitted to evaporate quickly the system must be sealed and become pressurized above atmospheric pressure the vapor pressure must be kept below 760 mm(hg)
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Biology, 21.05.2021 20:30



Mathematics, 21.05.2021 20:30

Mathematics, 21.05.2021 20:30



History, 21.05.2021 20:30


Mathematics, 21.05.2021 20:30






