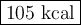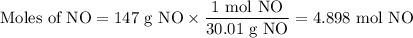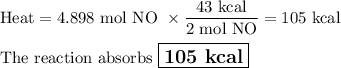Chemistry, 04.11.2019 07:31, elizabethwaller8104
How much heat is absorbed during production of 147 g of no by the combination of nitrogen and oxygen?
n2(g)+o2(g)→2no(g), δh = + 43 kcal/molhow much heat is absorbed during production of 147 g of no by the combination of nitrogen and oxygen?
n2(g)+o2(g)→2no(g), δh = + 43 kcal/mol

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, mimibear2932
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 23.06.2019 04:00, ayoismeisjjjjuan
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2

Chemistry, 23.06.2019 07:00, jaydenboi604
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample?
Answers: 1
Do you know the correct answer?
How much heat is absorbed during production of 147 g of no by the combination of nitrogen and oxygen...
Questions in other subjects:

History, 28.09.2019 16:30

Social Studies, 28.09.2019 16:30

Physics, 28.09.2019 16:30

Biology, 28.09.2019 16:30





Mathematics, 28.09.2019 16:30