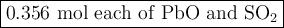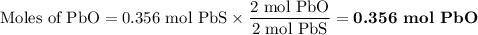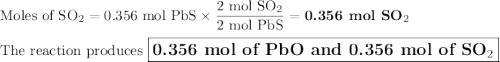2. for the following reaction, calculate how many moles of each product are formed when 0.356
...


Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 06:00, kylieweeks052704
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 09:00, miller5452
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1
Do you know the correct answer?
Questions in other subjects:


Mathematics, 22.01.2021 09:10

Mathematics, 22.01.2021 09:10

Mathematics, 22.01.2021 09:10


Computers and Technology, 22.01.2021 09:10

Mathematics, 22.01.2021 09:10


English, 22.01.2021 09:10

Biology, 22.01.2021 09:10