
Chemistry, 02.11.2019 07:31, morganhines181
Solution of the schrödinger wave equation for the hydrogen atom results in a set of functions (orbitals) that describe the behavior of the electron. each function is characterized by 3 quantum numbers: n, l, and ml. if the value of n = 1 the quantum number l can have values from to . the total number of orbitals possible at the n = 1 energy level is . if the value of l = 1 the quantum number ml can have values from to . the total number of orbitals possible at the l = 1 sublevel is .

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, maddyjones4172
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 14:00, cheyennemitchel238
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 14:30, Hannahmiller3773
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3
Do you know the correct answer?
Solution of the schrödinger wave equation for the hydrogen atom results in a set of functions (orbit...
Questions in other subjects:



Arts, 31.03.2021 19:50


Mathematics, 31.03.2021 19:50


Chemistry, 31.03.2021 19:50

Chemistry, 31.03.2021 19:50

Mathematics, 31.03.2021 19:50


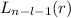 , being n and l the quantum numbers. As is known, in the Laguerre polynomials the subindex must be greater than or equal to 0, which implies
, being n and l the quantum numbers. As is known, in the Laguerre polynomials the subindex must be greater than or equal to 0, which implies 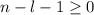 . So if n=1, the only possible value of l is l=0.
. So if n=1, the only possible value of l is l=0. ![Y_{l,m_l}(\theta,\phi)[\tex] as solution. One of the properties of spherical harmonics is that the number [tex]m_l[\tex] can only take the values [tex]m_l=-l,-l+1,-l+2,...,l-1,l[\tex]. So if l=0, as above, [tex]m_l[\tex] can only take the value [tex]m_l=0,[\tex] thus giving only 1 orbital. But if l=1, [tex]m_l](/tpl/images/0356/6953/a304c.png) can be
can be 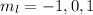 . Therefore the sublevel l=| has 3 possible orbitals.
. Therefore the sublevel l=| has 3 possible orbitals.



