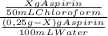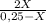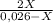Chemistry, 02.11.2019 06:31, vanessacox45
7. one gram of aspirin dissolves in 300 ml of water and in 17 ml of chloroform at room temperature. an aqueous solution of 0.25g aspirin (100 ml water) was extracted with 100 ml of chloroform. how much aspirin was recovered from the chloroform layer? what if instead, the aqueous solution was extracted twice, each time with 50 ml of chloroform, keeping the water amount at 100ml. how much total aspirin would be recovered from the two extractions?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 06:30, dpchill5232
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 10:10, andersonemma2222
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Do you know the correct answer?
7. one gram of aspirin dissolves in 300 ml of water and in 17 ml of chloroform at room temperature....
Questions in other subjects:

Mathematics, 25.02.2021 14:00



Medicine, 25.02.2021 14:00






Mathematics, 25.02.2021 14:00


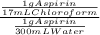 = 17,6
= 17,6