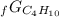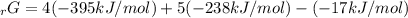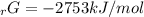Chemistry, 02.11.2019 05:31, WhiteMex69
Given the values of δgfo given below in kj/mol, calculate the value of δgo in kj for the combustion of 1 mole of butane to form carbon dioxide and liquid water. δgfo (c4h10(g)) = -17 δgfo (co2(g)) = -395 δgfo (h2o(l)) = -238

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:10, ChloeLiz7111
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 04:30, logan12345677885675
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 04:40, deedee363
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Do you know the correct answer?
Given the values of δgfo given below in kj/mol, calculate the value of δgo in kj for the combustion...
Questions in other subjects:


Mathematics, 16.10.2020 08:01

Mathematics, 16.10.2020 08:01



Mathematics, 16.10.2020 08:01




Social Studies, 16.10.2020 08:01



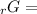 4Δ
4Δ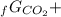 5Δ
5Δ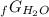 -Δ
-Δ