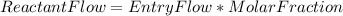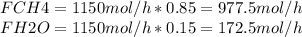Chemistry, 02.11.2019 04:31, Svetakotok
Steam reforming of natural gas is the most common method of producing commercial hydrogen. a stream with a flow rate of 1150 mol/h containing 85.0 mol% ch4 and 15.0 mol% of water is combined with additional water steam and fed to a steam reforming reactor to produce hydrogen. the stream coming out is in chemical equilibrium. the fractional conversion for both the water and methane are 0.600. balance the chemical equation and calculate how much additional water steam is fed to the steam reforming reactor and the flow rate of the outlet hydrogen. ch4 + h2o < --> co + 3h2 (balanced)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, bbyniah123
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 09:20, pandaman632
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 12:30, fvmousdiana
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
Do you know the correct answer?
Steam reforming of natural gas is the most common method of producing commercial hydrogen. a stream...
Questions in other subjects:

Mathematics, 26.06.2019 10:30

Mathematics, 26.06.2019 10:30


Computers and Technology, 26.06.2019 10:30

Mathematics, 26.06.2019 10:30




Health, 26.06.2019 10:30